Contribution to EU-legislation
How the obligation to list the ingredients on cosmetics came about

How it started
In the 1980s, when I was doing my PhD research on side effects of cosmetics, the lack of information on the ingredients in those products was a major problem. Whereas cosmetics manufacturers in the United States had been legally obligated since 1974 to list all ingredients of cosmetics on the product (ingredient labeling), this was not the case in the Netherlands and the other countries of the European Economic Community (EEC). During my research I had become convinced that such a requirement in the EEC would be very helpful for patients with cosmetic allergies, dermatologists, and consumers who, for whatever reason, are interested in the ingredients in cosmetic products. Labeling would enable the latter group to make a conscious and substantiated choice of cosmetics. Proposition 5 of my 1988 dissertation therefore read as follows: 'It is urgently necessary that the listing of all components of cosmetics on the packaging in the EEC is made legally mandatory'. That legal obligation would indeed come about, but only 9 years later, in 1997.
I believe – in all (im)modesty – that my dissertation and my subsequent activities to get labeling on the Brussels agenda played an important role in this. How did that work?
The problem
An important question in my PhD research was 'What are the allergens in cosmetics?' Allergens are the components of cosmetic products that cause an allergic reaction in a consumer/patient. A contact allergy is diagnosed with so-called patch tests, also known as epicutaneous allergy tests. A large number of substances, which are known to be able to cause allergic reactions, are applied to the back in small tubs made of plastic or aluminum and fixed with plasters. The materials are removed after 2 days (day 2, D2) and half an hour later the tests are 'read', to see if an allergic reaction has occurred. A second reading is taken one or two days later (D3 or D4), and if the procedures is performed properly, also on day 7, because some reactions develop very slowly. A positive allergic patch test reaction is characterized by itching, redness (erythema), swelling (oedema) and possibly vesicles or even a blister.

The allergens are placed in plastic tubs. On top of this comes another layer with sturdy plasters to fix everything well. The entire construction will remain in place for two days and the materials should not shift
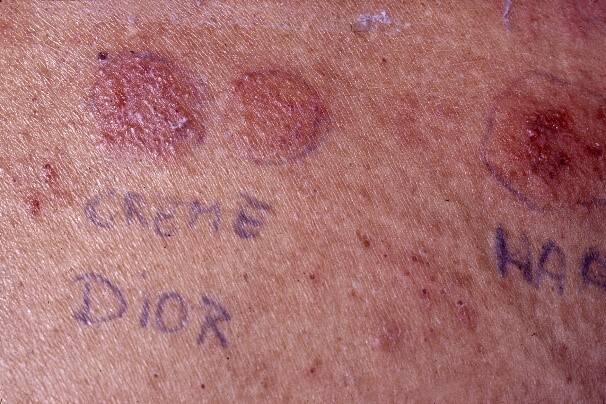
Beautiful ++ positive reactions to a cosmetic.
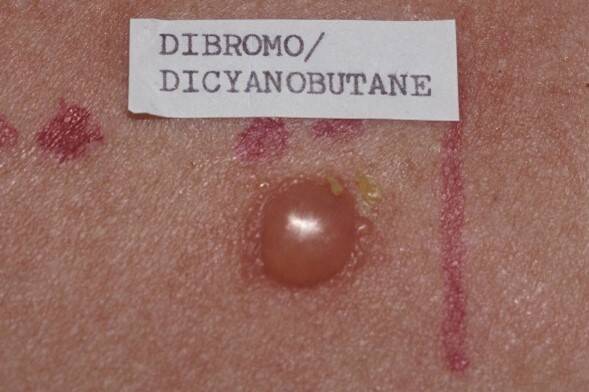
A +++ positive reactions with blister to the preservative methyldibromo glutaronitrile (dibromodicyanobutane).
In my research, both in my own practice and in the joint research with the members of the Contact Dermatoses Committee, patients were patch tested with their own cosmetics in addition to the European standard series and (possibly) a cosmetics series. When a positive reaction to the cosmetic was observed, the question was of course: which component is responsible? Sometimes, in addition to the reaction to the cosmetic, there would also be positive patch test reactions to one or more allergens in either the European standard series or the cosmetics series, which includes known allergens in cosmetics, such as fragrance raw materials, certain preservatives and base ingredients such as lanolin alcohol (wool alcohols). Could those be the cause?
Insufficient information about the ingredients
And that's where the problem loomed: we didn't know that, because – with the exception of 'perfume' (which you could smell) – we had no idea whether those substances were in it. And if, apart from the positive patch test on the cosmetic, there was no other positive reaction, we certainly had no idea which ingredient caused the allergic reaction. And why not? Because no information was available on the composition of the cosmetic product in question, neither on the product itself nor on any information leaflet or packaging. In such a case, we had to contact the manufacturer and kindly request information. Well, at that time many cosmetic manufacturers were still quite hostile to dermatologists, because they 'only say bad things about cosmetics' (only a few did, and I certainly didn't). In fact, many manufacturers or importers did not respond at all to requests for information, others responded only to a limited extent or only after much insistence.
Depending on the co-operation of the cosmetic industry
How could my question: 'What are the allergens in cosmetics' be answered? For that we depended on the cooperation of the cosmetics industry. I had found the Keuringsdienst van Waren (KvW) in Enschede, Cosmetics section (head: dr. D.H. Liem, later dr. J.W. Weijland) willing to participate in the study. When we had seen a patient with a proven cosmetic allergy (with a positive patch test reaction to the product), the KvW would contact the manufacturer or importer and asked not only for information on the ingredients, but also for the ingredients themselves. Once the KvW had received those components (which was by no means always possible, many refused and the manufacturers could not legally be forced to cooperate), they were diluted to the right concentration for patch testing in the appropriate solvent (usually petrolatum, sometimes water or alcohol) and then tested in patients with the cosmetic allergy. Then, if there were one or more positive patch test reactions to the ingredients, we knew which one(s) were the allergen(s) that had caused the allergic reaction in the patient. Thet patient was of course advised against using the product any more, otherwise an allergic reaction would occur again. However, other cosmetics might also contain that allergen, so these had to be avoided as well!

Allergic contact eczema of the eyelids due to tosylamide/formaldehyde resin in nail polish. This is called an 'ectopic' eczema, because it arises in a different place than where the cosmetic is applied. Moisture, for example when washing hands, causes some of the allergen to leak from the nail polish and scratching or rubbing causes it to end up on the eyelids, where it causes an allergic reaction.
Next problem
And there was the second problem. The patient now knew from the allergy tests to which chemical she was allergic. Obviously, we had instructed her to avoid this substance in cosmetics. But how could she do that? This was almost impossible, because no information on the ingredients of any cosmetic product was available to the consumer. As a consequence, she could not safely choose an alternative product. Identifying the allergen therefore had no practical consequences, except in the case of perfume allergy, in which case she could opt for unscented cosmetics. Here too, mandatory labeling would be the solution. The patient could then see on the packaging, which products did not contain 'her' allergen' and thus these cosmetics were safe to use. So I decided to make a serious effort to bring about this legislation for mandatory labeling in the Netherlands, and preferably in the entire EEC.
The road to legislation for mandatory labeling of cosmetics
And so, at some point in 1989, I went to national government representatives in Rijswijk to tell my story and convince them that legally required labeling would be very useful for dermatologists and patients and that the cosmetics industry would experience minor and only temporary disadvantages. Three government officials listened benevolently and asked some critical questions that I could answer without any problems. They complimented me on my initiative and we said goodbye in a positive atmosphere. I had a good feeling about it. Six weeks later I received an official letter, the contents of which can be summarized as follows: 'Sorry, Dr. De Groot, we don't feel like it, we don't think the subject is important enough to make any efforts' . First attempt failed.
I had another and maybe even better idea. I had read that a scientific European association had recently been founded aimed at contact allergy science: the European Society of Contact Dermatitis (ESCD). So I contacted the ESCD Board, explained the problem and the solution, and asked whether we could perhaps tackle this in a European context. My proposal was received with great enthusiasm and I was promptly appointed 'Convenor' of the ESCD's 'Working Party European Community Affairs'. The other members of the working group were An Goossens (Belgium), Peter Frosch (West Germany), Nicole Hunziker (Switzerland), Torkil Menné (Denmark), Antonella Tosti (Italy), and John Wilkinson (United Kingdom).
Position paper describing the problem and the solution
Our first goal was to publish a position paper in a major general medical journal to describe 1. who benefits from labeling; 2. how many people benefit; 3. what objections can be expected from the cosmetic industry; and 4. what problems can arise with the introduction of new legislation in the EEC. We described that labeling is useful to patients with allergies to cosmetics, to dermatologists, and to patients who do not have eczema from cosmetic products themselves, but who have been shown to be allergic to substances that are (also) used in cosmetic products. Moreovero, we argued, ingredient labeling will stimulate scientific research, and new contact allergens identified sooner by dermatologists thanks to the information, that has come available from labeling. Next, this data can then be used by the industry to make their products safer, thus ultimately benefiting not only patients and dermatologists, but also the industry itself.

The working group calculated that about 10% of all patients patch tested by dermatologists are allergic to cosmetics, and that about 35% of all patients tested with the European standard series are allergic to substances that may be present in cosmetics. Extrapolating we estimated that 2-3% of the general population is allergic to cosmetics or cosmetic ingredients. Therefore, we argued that the number of people benefiting from labeling is large enough to justify the time and expense required to adapt legislation in the EEC.
Objections of the cosmetics industry
We anticipated that the cosmetics industry would object to mandatory labeling. An important argument would be that manufacturers wanted to keep their 'valuable formulations' secret. We countered that argument by stating (which was true, by the way) that a good cosmetic chemist can copy all cosmetics by chemical analyses, even without a priori knowing what ingredients they contain. The legislator, we suggested, might make an exception for 'truly' secret and commercially important ingredients. And then of course there were objections to extra costs, less flexibility in product composition changes and loss of space on the packaging of small products. We argued that this would only entail minor and temporary disadvantages for the cosmetics industry. After all, the whole process had already taken place in the United States 15 years earlier, and the industry there subsequently flourished like never before.
The only practical problem we foresaw was the nomenclature for labeling, since Europe has many languages. We therefore proposed using the nomenclature developed by the US Cosmetics, Toiletry, and Fragrance Association (CTFA) for labeling cosmetic products. This had to be adjusted somewhat. Sunscreens, for example, did not have a CTFA name, because they were and are registered as drugs (medicines) in the USA. The European Commission would later adopt our proposal and two largely overlapping nomenclature systems were developed: INCI EU and INCI USA, in which INCI stands for International Nomenclature Cosmetic Ingredients.
Position paper in British Medical Journal
We submitted our article (the position paper) to the British Medical Journal (BMJ) and it was accepted with minor changes: De Groot AC. Labeling cosmetics with their ingredients. Brit med J 1990;300:1636-1638. Of course I had listed all members of the working group as co-authors, but the editors of the BMJ decided to mention my name only as author and the names of the others at the end of the article as 'members of the Working Party', on whose behalf I had written the article. Not elegant, in my opinion, but not my decision. Fortunately, nobody blamed me for it, fortunately.

Our position paper in the British Medical Journal, published in 1990
Lobbying
With this position paper in hand, all members of the working party started lobbying and we approached government officials, consumer organizations, medical organizations, the cosmetics industry, newspapers and television in our respective countries. Remarkably, from my practice room at Carolus Hospital, I made it to German television, debiting auf Hochdeutsch: 'Wir Dermatologen erfahren immer viel zu spät, was die Kosmetikindustrie für neue Inhaltsstoffe verwendet ……'. In Brussels, An Goossens and I think also Ian White (who joined later and turned out to be very important for the cause) actively campaigned in legislative organizations. Long story short: in 1991, the Commission of the European Communities drafted new legislation that made ingredient labeling mandatory for all cosmetic products sold in the European Community. An exception was made for the fragrance materials in perfumes and fragranced products. That made perfect sense: A perfume can contain many dozens to 100 ingredients and it is impossible to label them all. The presence of fragrances was to be identified by 'perfume' or 'aroma' in the ingredient list. Incidentally, it would become mandatory in 2005 to label the presence of 26 different fragrance ingredients in cosmetics and household products (De Groot AC. Contact allergy to fragrance ingredients. 2. Recent developments. Ned Tijdschr Derm Venereol 2010;20:382-387).
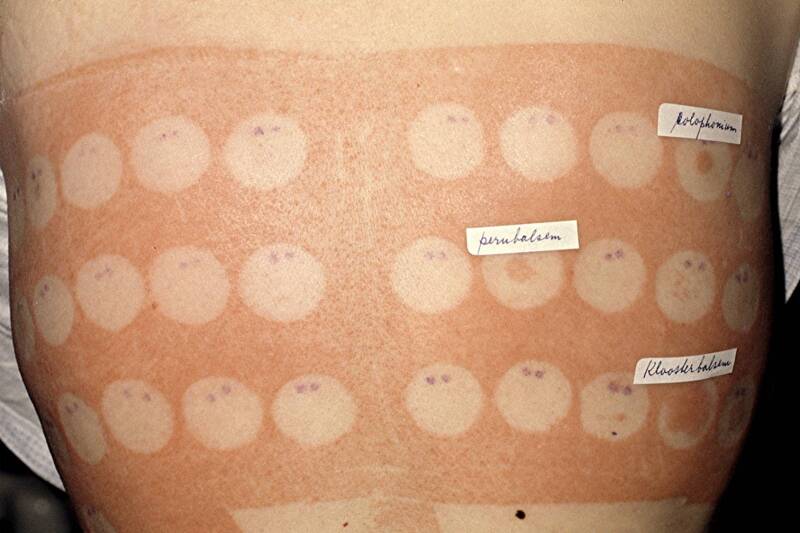

The adhesive plasters that were used in the past to secure the patch test materials sometimes caused an allergic reaction themselves. In such cases, the entire skin that had been in contact with the material was found to be red when removing the patch test materials. Only the skin of the patch test-negative allergens remained normal. The most frequent allergen in plasters back then was colophony (rosin), a well-known Pine tree-derived material. The adhesive plaster in the right image also contained colophony and indiced a classic allergic reaction. Nowadays, reactions to adhesive plasters for patch testing are very rare, colophony is hardly used anymore in these materials or band aids.
Mission accomplished
The legal requirement for ingredient labeling throughout the European Economic Community became effective December 31, 1997, giving the cosmetics industry ample opportunity to prepare for and implement the new requirements. Our mission was accomplished!
In several articles, which are listed below (which shows that I was not averse to double or even triple publications), I have, sometimes together with other authors, informed dermatologists, general practitioners and pharmacists of the impending changes. It was important that they were familiar with the INCI nomenclature and could look up new names, as many familiar names would change. Wool alcohols turned into lanolin alcohol and Peru balsam into Myroxylon pereirae resin, just to give two examples. All plant products were given a different name, because the botanical names are used in the INCI nomenclature. Bergamot oil, for exampl,e became Citrus aurantium bergamia fruit oil and geranium oil became Pelargonium graveolens oil. Well, that takes some getting used to….. That is why I published conversion lists from old to new names, which could also be handed out to patients. After all, the new names of the INCI nomenclature would from now on be mentioned in the ingredients list on the cosmetic product, the packaging or a label.
Labeling has now existed in the European Union for over 25 years and all our predictions have come true: great benefits for allergic patients, dermatologists and consumers and hardly any and only temporary disadvantages for the cosmetics industry.
- De Groot AC. Labelling cosmetics with their ingredients. Brit med J 1990;300:1636-1638
- De Groot AC. Ingrediëntendeclaratie op cosmetica. Apotheek in Praktijk 1990 (december), 14-18
- De Groot AC. Ingrediëntendeclaratie op cosmetica. Bulletin Contactdermatosen 1990;4:158-162
- De Groot AC, White IR. Cosmetic ingredient labelling in the European Community (Editorial). Contact Dermatitis 1991;25:273-276
- De Groot AC, Weijland JW. Conversion of common names of cosmetic allergens to the INCI nomenclature. Contact Dermatitis 1997;37:145-150
- De Groot AC, van Ginkel CJW, Weijland JW. Ingrediëntendeclaratie op cosmetica: de nieuwe EU-richtlijnen. Ned Tijdschr Derm Venereol 1997;7:154-157
- De Groot AC, van Ginkel CJW, Weijland JW. Ingrediëntenvermelding op cosmetica. Ned Tijdschr Geneeskd 1997;141:1747-1748
- De Groot AC, van Ginkel CJW, Weijland JW. Ingrediëntendeclaratie op cosmetica: de nieuwe EU-richtlijnen. Signa 1998;27:14-16
- De Groot AC, van Ginkel CJW, Weijland JW. Hulp voor dermatologen en patiënten. Ingrediëntendeclaratie op cosmetica: de nieuwe EU-richtlijnen. Pharmaceutisch Weekblad 1999;134:132-135

